“This is an evolutionary fight…”
That’s what Ken Frazier – chairman and CEO of pharmaceutical giant Merck – said on live television back in March.
At the time, COVID-19 had already spread globally. The World Health Organization had just labeled the virus as a “pandemic.” There were more than 190,000 confirmed cases with 7,807 deaths.
So it may surprise us to know that Frazier wasn’t talking about COVID-19.
Frazier was referring to a far more serious global health crisis. It’s a crisis that kills an estimated 700,000 people every year. By 2050, that number could climb as high as 10 million.
Right now, the world is focused almost exclusively on COVID-19. But there is another pandemic bubbling beneath the surface.
Evolutionary Fight
As I’ve been showing my readers, there are several reasons to be optimistic about COVID-19.
Just last week, I shared new research from the Stanford University School of Medicine that showed why the actual COVID-19 mortality rate is likely on par with seasonal flu and possibly much lower.
But while the world gets COVID-19 under control, other global health crises are developing.
Ken Frazier – quoted above – was speaking about something entirely different when he said, “This is an evolutionary fight.”
Here’s Frazier:
With these kinds of viral outbreaks, severe infections are often accompanied by bacterial infections, sometimes super-bacterial infections. You go back a decade to H1N1, nearly half of the 300,000 people who lost their lives died because of bacterial pneumonia. It’s important that we continue to develop new, powerful antibiotics to ensure that – with respect to this outbreak and future outbreaks – we have those things in our arsenal.
[… ]
We want to make sure that we understand that this is an evolutionary fight between us and the bacteria.
When Frazier references “super-bacterial infections,” he’s referring to bacteria that are resistant to today’s antibiotics. In the medical industry, this is referred to as antimicrobial resistance. We may also know these bacterial infections as “superbugs.”
As I mentioned above, it’s estimated that there are 700,000 deaths each year as a result of these super-bacterial infections. And that number is expected to hit 10 million by 2050.
And keep in mind, that estimate was presented before the world encountered COVID-19. This problem is far more deadly than COVID-19 will ever be. And because of this new coronavirus, the spread of superbugs is likely to speed up.
“Nightmare Scenario”
One of the most common side effects for patients who contract COVID-19 is bacterial pneumonia. In one study published by Lancet, of 41 hospitalized COVID-19 patients, 10% developed secondary infections.
And physicians are prescribing more antibiotics than normal to stop these infections.
Dr. Priya Nori – who works at a Bronx-based hospital – recently said, “We tend not to hold back on antibiotics in [COVID-19] patients.”
As more antibiotics are prescribed, bacteria become more resistant. And the problem of superbugs grows larger.
To make matters worse, intensive care units are hotbeds for antimicrobial resistance. And in viral hot spots like New York City ICUs, it’s suspected that the spread of COVID-19 is also making the problem of superbugs more prevalent.
Bo Shopsin, an infectious disease expert from New York University’s Langone Health Center, put it this way: “It’s quite clear that COVID is transmitting in hospitals. And if it is, [resistant bacteria] are too.”
Neil Clancy – an infectious disease physician from the University of Pittsburgh – put it even more simply: “In terms of a nightmare scenario, it’s quite scary.”
Void of Discovery
We may be wondering, how is this possible?
I’m certain that everybody reading this today has taken antibiotics at one time in their lives. And for much of recent history, these antibiotics have worked well enough.
Since the discovery of penicillin in 1928, the world saw an explosion of scientific discoveries in antibiotics. One after the other, scientists discovered new forms of antibacterial drugs to fight off bacterial infections… until 1987.
Then, there was a void where no new advancements were made… one that’s lasted more than 30 years.
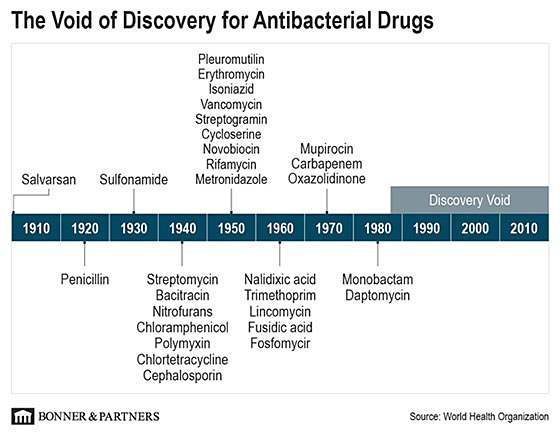
And we can see the consequences for ourselves…
Today, a class of antibiotics called quinolones (more specifically fluoroquinolones) is one of the most widely used antibiotics worldwide.
Over the last 15 years, bacteria have become resistant to quinolones. Quinolone antibiotics just don’t work as well as they used to.
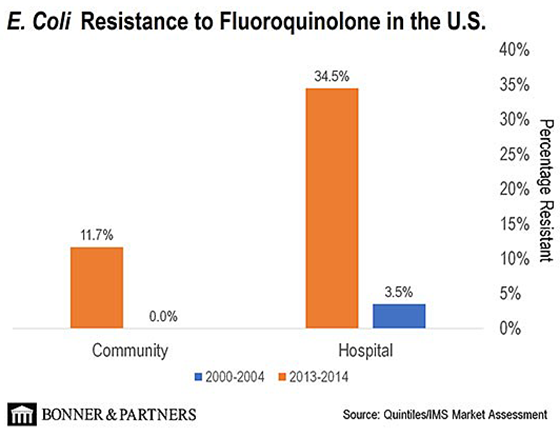
In the 2000–2004 timeframe, bacterial resistance to treatment using quinolones was only 3.5%. That basically means that 96.5% of patients were able to recover using quinolone treatment.
But in the 2013–2014 timeframe, resistance levels jumped to 34.5%. Only 65.5% of patients were able to recover using quinolones. In short, the bacteria have been developing a resistance to quinolones… And the trend continues today.
Throughout all my research in high technology, I’ve never seen this kind of “void of discovery” before. I can’t think of any other example of technology that has shown virtually no significant progress over the last 30 years.
But this also presents an interesting opportunity…
The Company Ending the Void of Discovery
The world seems almost singularly focused on beating COVID-19. But behind the scenes, the world’s next public health crisis is already taking shape. Fortunately, I believe I’ve identified the one biotech company positioned to stop it.
This company’s lead product candidate is designed to treat a very specific type of antibiotic-resistant bacteria. This type of bacteria is responsible for about 400,000 hospitalizations in the U.S. each year. And it comes at an incredible annual cost of $2.8 billion.
The company believes that the opportunity in Europe is about 60% of the potential U.S. business, which would be an additional $2.58 billion. In total, for just the U.S. and Europe, we’re looking at a potential $6.88 billion opportunity.
In July 2018, this biotech announced positive results for its Phase 1 clinical trials. And get this: This drug has been used for many years in Japan with few adverse side effects.
Because of this, the FDA granted the drug a qualified infectious disease product designation. In other words, the FDA allowed the company to skip Phase 2 trials and move directly into Phase 3.
The trial results for Phase 3 are expected in Q3 of this year. And when approved, it will be the only oral antibiotic of its kind in the market. It will also have zero competition from generic drugs, and the company’s intellectual property (patents) are protected through 2038.
And at these levels, I’m predicting investors could see returns above 1,000%.
This is a story I’ve been wanting to share with my readers for some time. And with COVID-19 highlighting the need for new antibacterial therapies, I believe now is the right time.
Go right here for the full story.
Regards,
Jeff Brown
Editor, Exponential Tech Investor